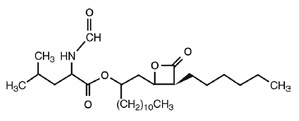
Chemical Structure of Orlistat
 Orlistat is approved for the management of obesity, including weight loss and weight
management when used in conjunction with a reduced-calorie diet; reduce the risk of weight
regain after prior weight loss; indicated for obese patients with an initial body mass index >30 kg/m2 or >27 kg/m2 in the presence of
other risk factors. Orlistat is approved for the management of obesity, including weight loss and weight
management when used in conjunction with a reduced-calorie diet; reduce the risk of weight
regain after prior weight loss; indicated for obese patients with an initial body mass index >30 kg/m2 or >27 kg/m2 in the presence of
other risk factors.
------------------------------------------------------------------------------------------------------------
Clinical Pharmacology
Orlistat is a potent, specific, irreversible inhibitor of pancreatic and gastric lipases. Also known as tetrahydrolipstatin, it is a chemically synthesized derivative of lipstatin, which is naturally produced by Streptomyces toxytricini. It exerts its pharmacologic activity by forming a covalent bond with the active serinesite of gastric and pancreatic lipases in the lumen of the gastrointestinal tract. This action prevents these enzymes from hydrolyzing dietary fat (in the form of triglycerides) into absorbable free fatty acids and monoglycerols. Undigested triglycerides are eliminated by the fecal route. Lipase inhibition induced by orlistat decreases systemic absorption of dietary fat, thereby contributing to caloric deficit. The agent does not appear to inhibit the activity of other  pancreatic enzymes, such as phospholipase A2, amylase, or trypsin. pancreatic enzymes, such as phospholipase A2, amylase, or trypsin.
Data from pharmacokinetic studies using radiolabeled orlistat in both non-obese and obese individuals indicate that the drug is not appre-ciably absorbed into the systemic circulation.
After single oral doses of C-orlistat 50 or 360 mg, maximum plasma concentrations of total radioactivity occurred within approximately 8 hours. At the time of peak total radioactivity, plasma concentrations of orlistat were below the detectable assay limit of 5 ng/ml. In multiple-dose studies, using a more sensitive assay with a lower limit of detection equal to 0.20 ng/ml, detection of orlistat in human plasma was infrequent, with low measurable concen-trations ranging from 0.20-8.8 ng/ml. There was also no evidence of drug accumulation in patients treated with a dosage of 120 mg 3 times/day for up to 2 years. Thus all of orlistat’s pharmacologic actions are believed to be exerted in the gastrointestinal tract.
------------------------------------------------------------------------------------------------------------
Pharmacokinetics
Metabolism of the agent appearsto occur in the gastrointestinal wall. Based on data 1from studies of radiolabeled orlistat in obese patients, two major metabolites were identified, M1, with a hydrolyzed 4- member lactone ring, and M3, M1 with the N-formal leucinemoiety cleaved. These metabolites accounted for approximately 42% of total radioactivity in plasma, with mean concentrations of 26 ng/ml and 108 ng/ml for M1 and M3, respectively, 2-4 hours after drug administration. 
Both M1 and M3 contain an open b-lactone ring in their chemical structures. Due to their very low plasma concentrations, and given data indicating that the b-lactone ring is required for lipase inhibition, they are considered to be pharmacologically inactive.
Orlistat is excreted almost completely by the fecal route. After an oral dose of C-orlistat 360 mg in normal-weight and obese subjects, approximately 97% of drug was recovered in feces, with 83% as unchanged drug. Less than 2% was recovered in urine, and complete excretion of total radioactivity occurred 3-5 days after administration.
------------------------------------------------------------------------------------------------------------
Dosage

120mg three times daily with each main meal.
(During or upto 1 hour after meal).
The patient should be on nutritionally balanced and reduced calorie diet. The daily intake of fat, carbohydrate and protein should be distributed over three main meals, if the meal is occasionally missed, the dose of Orlistat can be omitted.
------------------------------------------------------------------------------------------------------------
Adverse Effects
Gastrointestinal disturbances, including faecal urgency and incontinence, flatulence, and fatty stools or discharge, are the most frequently reported adverse effects during treatment with Orlistat. They may be minimised by limiting the amount of fat in the diet.
------------------------------------------------------------------------------------------------------------
Precautions
 Orlistat should not be given to patientswith chronic malabsorption syndrome or cholestasis and should be given with caution to patients with a history of hyperoxaluria or calcium oxalate nephrolithiasis. Adjustments to dosage of hypoglycaemics may be necessary in patients with type II diabetes because of improved metabolic control after weight loss in these patients. Supplements of fat-soluble vitamins may be necessary during long-term therapy, but they should be taken at least 2 hours before or after an orlistat dose or at bedtime. Orlistat should not be given to patientswith chronic malabsorption syndrome or cholestasis and should be given with caution to patients with a history of hyperoxaluria or calcium oxalate nephrolithiasis. Adjustments to dosage of hypoglycaemics may be necessary in patients with type II diabetes because of improved metabolic control after weight loss in these patients. Supplements of fat-soluble vitamins may be necessary during long-term therapy, but they should be taken at least 2 hours before or after an orlistat dose or at bedtime.
------------------------------------------------------------------------------------------------------------
Interactions
Orlistat may reduce the absorption of fat-soluble vitamins. In patients taking warfarin, international normalised ratio should be monitored during treatment with orlistat. A reduction in cyclosporin concentrations to subtherapeuticlevels has been reported in transplant recipients given orlistat.
------------------------------------------------------------------------------------------------------------
Uses and Administration
Orlistat is a gastric and pancreatic lipase inhibitor that limits the absorption of dietary fat. It is used together with dietary modification in the management of obesity i.e. in patients with a BMI of 30 kg/m2 or greater. 
It may also be used in overweight patients with a BMI of 27 kg/m2 or more if there are associated risk factors. Orlistat is given in a usual dose of 120 mg by mouth three times daily, immediately before, during, or up to 1 hour after meals.
If a meal is missed or contains no fat, the dose should be omitted. Orlistat therapy should be stopped if the patient does not lose at least 5% of their body-weight during the first 12 weeks of therapy. |